ABOUT US
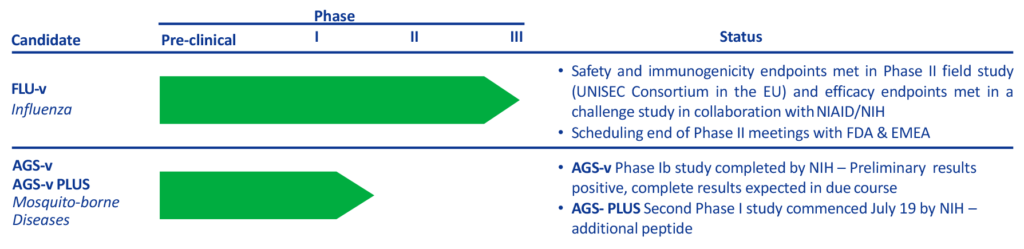
Imutex Limited, formed in 2016, is a joint venture between PepTcell and hVIVO, to accelerate the development of a Broad-Spectrum ‘Universal’ Influenza Vaccine, FLU-v, and a Mosquito-Borne Diseases Vaccine, AGS-v.
The development of the FLU-v Universal Flu Vaccine and the AGS-v Mosquito-Borne Diseases vaccines are key public health priorities identified by the Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) in the United States.
Imutex is collaborating with the National Institute of Allergy and Infectious Diseases (NIAID) to accelerate development of both vaccines.
Learn more about AGS-v here and FLU-v here.
Background information on Universal and Annual Flu vaccines can be found at www.endfluenza.com
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |


